Introduction
Chimeric antigen receptor T-cell therapy (CAR T) is approved as second-line therapy in patients with large B cell lymphoma (LBCL) that is refractory to, or relapsed within 1 year of, initial therapy. In patients with LBCL that relapses more than 1 year after completion of initial therapy, salvage chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) remains the standard of care for transplant-eligible patients. However, data on the outcomes of salvage chemoimmunotherapy with intent for ASCT for this specific population are lacking.
Methods
We retrospectively reviewed patients with relapsed LBCL more than 1 year after 1 st line therapy who received salvage treatment. We included patients seen at our institution prior to the start of salvage therapy, who began therapy between 1/2008 and 1/2023, and were treated with stated intent to proceed to ASCT. We excluded patients with CNS involvement at relapse and those not evaluable for response. The primary outcome was progression-free survival (PFS), defined as the time from initiation of salvage therapy to LBCL progression or death. Event-free survival (EFS) was defined as time from salvage initiation to change in lymphoma therapy, disease progression, or death. Additional key outcomes included overall survival (OS), overall response rate (ORR) and complete response rate (CRR) to salvage therapy, and outcomes after subsequent CAR T. Outcomes for important subgroups using baseline characteristics were compared. ORR and CRR were compared using Fisher's exact test. PFS, EFS and OS were estimated using the Kaplan-Meier method, and differences were tested by the log-rank test.
Results
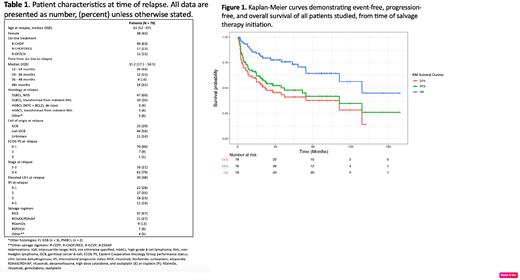
A total of 78 patients met inclusion criteria, with characteristics summarized in Table 1. Median time from completion of initial therapy to relapse was 31.2 months (range, 12.3 - 177 months). CRR to salvage therapy was 76%, with a 94% ORR. In 60 patients who completed ASCT, CRR was 85%. With a median of 45 months' follow-up from salvage initiation, median PFS was 50 months (95% confidence interval (CI) of 25 - NR), and median EFS was 29.2 months (95% CI, 19.6 - NR)( Figure 1). Median OS was 132 months (95% CI 108 - NR). On subgroup analysis, factors significantly associated with shorter PFS were time from last treatment to relapse < 36 months, Ann Arbor stage 3-4, LDH above normal, and increased international prognostic index at relapse. Other factors such as age, gender, salvage regimen, extranodal disease, cell of origin, transformation from indolent lymphoma, bulky disease, and marrow involvement at relapse, were not significantly associated with PFS. Comparing response to salvage in 60 ASCT recipients, patients in CR (n=52) had longer median PFS than those in PR (n=8) (108 vs 33 mo), but this was not statistically significant (p = 0.4).
In 33 patients who received 3 rd line therapy or beyond, 16 (48%) received CAR T therapy; 7 proceeded directly to CAR T after inadequate response to salvage, and 9 had progression after completion of ASCT and received CAR T. Among all patients, CRR after CAR T was 81% and ORR 94%, with median PFS and OS not reached; PFS and OS at 24 months after CAR T were 63% and 81%, respectively.
Discussion
To our knowledge, this is the first study to specifically evaluate outcomes of ASCT-eligible patients with relapsed LBCL more than 1 year after initial therapy, who did not meet FDA criteria for CAR T-cell therapy. We observed highly favorable outcomes, with longer PFS and OS compared to studies that evaluated late LBCL relapses (Hilton LK., JCO 2023, Harrysson S., BJH 2022), possibly reflecting the exclusive use of modern salvage regimens with intent for ASCT, improvements in subsequent therapies, or bias related to the single-center design. In patients who received CAR T in 3 rd line or later, frequent and durable responses were seen. However, due to the small sample size, whether CAR T should be considered as an alternative to ASCT in patients with late LBCL relapse remains unknown. Our data represent a benchmark for patients with LBCL relapses >12 months after completion of frontline therapy and may help inform patient counseling and future clinical trial designs.
Disclosures
Shah:Beyond Spring: Research Funding; BMS: Research Funding; ArcellX: Other: DSMB; Amgen: Research Funding; Janssen: Research Funding. Falchi:Genmab: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Genentech: Consultancy, Other: Advisory Board, Research Funding; Abbvie: Consultancy, Other: Advisory Board, Research Funding; Seagen: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board; AstraZeneca: Consultancy. Ghione:Kyowa Hakko Kirin: Consultancy; AstraZeneca Pharmaceuticals: Consultancy; Kite, A Gilead Company: Research Funding; Secura Bio: Consultancy. Hamlin:ADC Therapeutics: Consultancy. Horwitz:Yingli Pharma Limited: Consultancy; Tubulis: Consultancy; Affimed: Research Funding; Trillium Therapeutics: Consultancy, Research Funding; SecuraBio: Consultancy; ONO Pharmaceuticals: Consultancy; Verastem/SecuraBio: Research Funding; Millenium: Research Funding; Auxilius Pharma: Consultancy; Abcuro Inc.: Consultancy; Cimieo Therapeutics: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Celgene: Research Funding; ADC Therapeutics: Research Funding; Seattle Genetics: Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Crispr Therapeutics: Research Funding; Takeda: Consultancy, Research Funding. Johnson:Myeloid Therapeutics: Consultancy. Kumar:Abbvie Pharmaceuticals: Research Funding; Beigene: Research Funding; Celgene: Research Funding; Adaptive Biotechnologies: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding; BridgeBio: Current equity holder in publicly-traded company; Janssen: Consultancy; Kite Pharma: Consultancy; Loxo/Lily Oncology: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Lahoud:MorphoSys Inc, Kite: Consultancy. Lue:OncLive: Consultancy; Merck: Consultancy. Moskowitz:Seattle Genetics: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; Beigene: Research Funding; Merck: Honoraria, Research Funding; ADC Therapeutics: Research Funding. Palomba:BMS: Honoraria; Rheos: Honoraria; MustangBio: Honoraria; GarudaTherapeutics: Honoraria; Novartis: Honoraria; Pluto Immunotherapeutics: Honoraria; Juno: Honoraria, Patents & Royalties; Ceramedix: Honoraria; Smart Immune: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Synthekine: Honoraria; Thymofox: Honoraria; Cellectar: Honoraria; Kite: Honoraria. Torka:Seagen: Consultancy; Genentech: Consultancy; Lilly USA: Consultancy; ADC Therapeutics: Consultancy; Genmab: Consultancy; TG Therapeutics: Consultancy. Vardhana:Koch Disruptive Technologies: Consultancy; Immunai: Consultancy. Zelenetz:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; MEI Pharma Inc: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; SAB: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Honoraria; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Abbvie: Research Funding; None other than mutual funds (401K): Current equity holder in publicly-traded company. Scordo:Angiocrine Bioscience, Inc.: Research Funding; Medscape, LLC: Honoraria; Omeros Corporation: Consultancy, Research Funding; CancertNetwork (Intellisphere LLC): Honoraria; Amgen, Inc.: Research Funding. Perales:Vor Biopharma: Consultancy, Honoraria; Adicet: Honoraria; Kite: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Allogene: Research Funding; DSMB: Other; NexImmune: Consultancy, Current equity holder in publicly-traded company; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Sellas Life Sciences: Consultancy; Servier: Other; Cidara Therapeutics: Consultancy, Other; Medigene: Consultancy, Other; Merck: Consultancy, Honoraria; Syncopation: Honoraria; Caribou: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; AbbVie: Consultancy, Honoraria; Allovir: Consultancy; Astellas: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Celgene: Honoraria; MorphoSys: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Miltenyi Biotec: Honoraria. Salles:AbbVie: Consultancy, Honoraria; BMS/Celgene: Consultancy; BeiGene: Consultancy; Ipsen: Consultancy, Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Kite/Gilead: Consultancy; Janssen: Consultancy, Research Funding; ATB Therapeutics: Consultancy; Loxo/Lilly: Consultancy; Debiopharm: Consultancy; Nurix: Consultancy; Orna: Consultancy; Novartis: Consultancy; Molecular Partners: Consultancy; Genmab: Consultancy; Incyte: Consultancy; Nordic Nanovector: Consultancy; Owkin: Current holder of stock options in a privately-held company; EPIZYME: Consultancy; Merck: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal